What is radiation?
All matters are made up of tiny units called atoms. As radiation is mainly released from atoms, the first step to understand radiation is to know more about their structure and properties:
Structure of atom
All matters are made up of tiny units called atoms. Every atom has a nucleus which is surrounded by a cloud of electrons. The nucleus consists of uncharged neutrons and positively charged protons. Negatively charged electrons travel around the nucleus in their orbits, similar to the way planets moving around the sun.
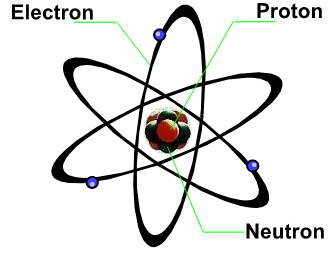
The numbers of protons and electrons in an atom are normally the same, giving an uncharged atom. The nucleus of the smallest atom - the hydrogen atom, contains only one proton. Whereas those of the bigger ones contain large numbers of protons and neutrons. For example, the carbon-12 nucleus contains 6 protons and 6 neutrons. The uranium-238 nucleus contains 92 protons and 146 neutrons.
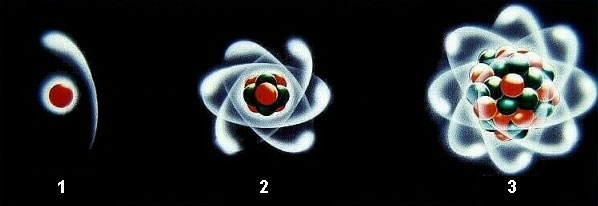
Atoms with different number of neturons, protons and electrons.
In picture: (1) Hydrogen (2) Carbon (3) Uranium
Nuclei of most atoms are stable (i.e. tend to remain as they are); but some nuclei, in particular, those large ones, are unstable. These unstable nuclei may release particles or electromagnetic waves spontaneously to return to their stable states. This process is called decay. The unstable nuclei are said to be radioactive and called radionuclides. The released particles and electromagnetic waves are called radiation.
Understand radiation
Radiation is everywhere in the universe. Since the inception of time, lives on earth have been exposed to radiation in the natural environment.
Radiation cannot be heard, seen, smelt nor tasted. Most of it cannot be felt. However, with the use of instruments, it can be detected and measured.