Ionizing radiation
Ionizing radiation includes high speed particles and high energy electromagnetic waves. Their energy is high enough to remove orbital electrons from atoms, thus giving rise to positively charged ions and negatively charged electrons.
Apart from x-rays which are emitted by excitation of electron clouds, most of the ionizing radiation come from the decay of unstable nuclei. Brief descriptions of different types of ionizing radiation are given below.
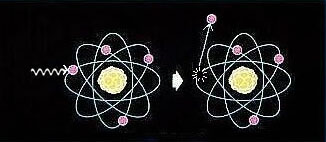
Ionization process
Alpha (α) particles
α particles, identical to helium nuclei, are positively charged particles consisting of two protons and two neutrons. As they are positively charged, their motion will be affected by an electromagnetic field. In general, heavy atoms (having atomic numbers greater than 82) would tend to emit α particles. Uranium and radium are examples of a emitting nuclei.
As α particles are relatively large and positively charged, they ionize matter easily and lose their energy very quickly. The penetrating power of α particles is the lowest among all types of ionizing radiation. α particles can be stopped easily by the outer layer of skin or a sheet of paper.
However, if α particles are taken into our body by inhalation or ingestion, such as inhaling part of a radioactive plume, α particles can directly affect tissues in the body. Although their penetrating power is weak, their ionization power is strong. The severity of biological effects inflicted will not be lower than other radiations.

A sheet of paper can stop α particles
Beta (β) particles
β particles are high speed electrons. Being charged, they are affected by an electromagnetic field. As they are much smaller than α particles, β particles have greater penetrating power. A sheet of aluminium a few millimetres thick can stop them. Many radioactive materials will emit β particles when they decay.
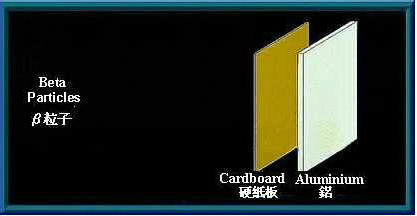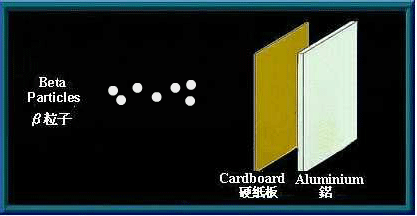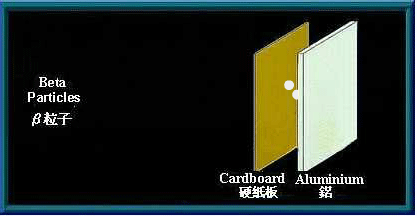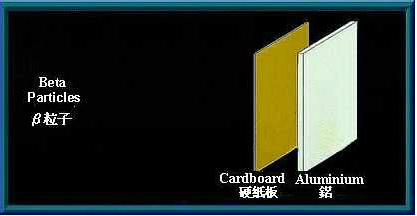
A sheet of aluminium can stop β particles
Gamma (γ) rays and x-rays
γ rays and x-rays are high energy electromagnetic waves. They have no mass and no electrical charge. They travel in straight lines in an electromagnetic field. Energy is transmitted in the form of electromagnetic waves similar to visible light, except that they have higher frequency and energy. γ rays and x-rays have great penetrating power and can pass through human body. Thick barriers of lead or concrete can stop them.
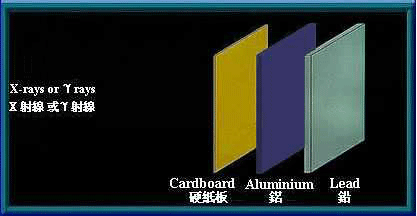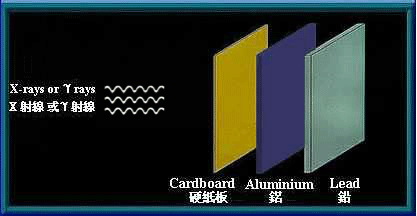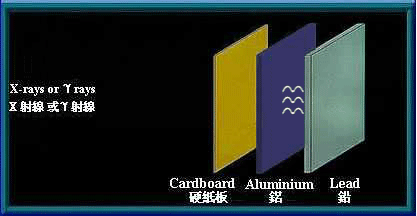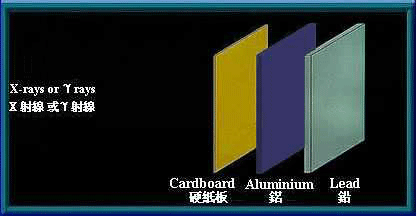
Thick barriers of lead can stop γ rays and x-rays
γ rays and x-rays are similar, the major difference is their origins. γ rays are emitted from the nuclei of unstable atoms during radioactive decay, while x-rays are from the electron cloud as the result of electron excitation.
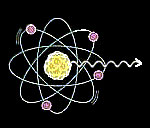
Origins of γ rays: γ rays are emitted from the nuclei of unstable atoms during radioactive decay

Origins of x-rays: x-rays are from the electron cloud as the result of electron excitation
Neutrons
Neutrons have no charge and are constituents of the atomic nucleus. They are very penetrating and can only be stopped by hydrogen-rich materials, such as water or paraffin. Fast-moving neutrons are produced by fission reaction in a nuclear reactor. Water is commonly used to moderate and control the speed of neutrons.

Paraffin can stop neutrons