Unstable nuclei
The stability of a nucleus can be described in terms of a number of parameters. One such parameter is the binding energy of particles inside the nucleus, i.e. the nucleons. The total binding energy of a nucleus is defined as the energy that would be required to separate the nucleus into separate nucleons. In other words, the same amount of energy will be given off when stand-alone nucleons come together to form the nucleus. When the binding energy per nucleon is plotted against the mass number, i.e. total number of protons and neutrons in a nucleus (the bottom right figure), it is found that the curve peaks around the mass number 56, i.e. the iron nucleus. This indicated that total energy of the iron nucleus is the lowest. In term of energy, nuclei larger than that of iron will have a tendency to break up while smaller nuclei would have a tendency to combine to form larger nuclei.
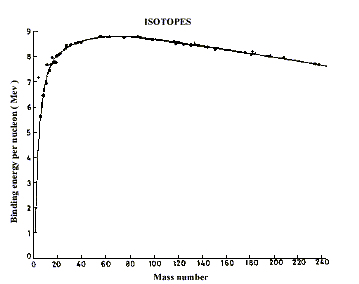
Binding energy per nucleon as a function of mass number (total number of protons and neutrons)
Uranium-235 is the fuel for nuclear power stations. It contains 92 protons and 143 neutrons. Because of the unstable nuclei, uranium has a tendency to break up and release a large amount of energy.
Unstable nuclei