A Brief Discussion on Gamma Radiation Survey Instruments
A Brief Discussion on Gamma Radiation Survey Instruments
LEUNG Ho-ming and LEE Shuk-ming
September 2025
When talking about radiation detection instruments, your first impression may be of an instrument that often appears in movies, TV series or games which makes a "click-click" sound. The more frequent the "click-click" sound or the greater the reading of the instrument, the more aware you may be that it implies a higher radiation level. In some video games with radiation as the theme, the player can take a "radiation antidote" to restore health even if the player has absorbed radiation doses. Such "radiation antidote" is just a fictional prop in a video game and does not exist in reality. However, the radiation detector that makes the "click-click" sound is real and is one of the most widely used radiation detectors.
Geiger–Müller Counter
The instrument that makes the "click-click" sound mentioned earlier is technically called a Geiger–Müller counter. In reality, Geiger–Müller counters are used to detect ionizing radiation, such as α particles, β particles, and gamma rays. Geiger–Müller counters measure the number of ionizations, and some counters will make a "click" sound to indicate an occurrence of an ionization. When there are more occurrences of ionizations, the "click" sound will be more frequent, and this means a higher radiation level. An ordinary Geiger–Müller counter can only measure the number of ionizations. If you want to measure the radiation dose rate, you need to use a specially designed energy-compensated Geiger–Müller counter which is properly calibrated. Because Geiger–Müller counters are lighter than other types of radiation detection instruments, they are more convenient for measuring environmental radiation dose rates. The Observatory uses a portable energy-compensated Geiger–Müller counter to measure the environmental gamma dose rate at survey sites. In natural environment such as rocks and soil, there are natural radionuclides. Some natural rocks (e.g. granite) contain more natural radionuclides than soil. For instance in Figure 1 we can see that count rates over grass measured by a portable energy-compensated Geiger–Müller counter are lower than count rates over rocks, indicating that the latter contain more natural radionuclides. In addition to using portable Geiger–Müller counter, the Observatory has also installed a fixed energy-compensated Geiger–Müller counter on the roof of radiological survey vehicle (Figure 2: "Gamma Dose-rate Meter" installed on the roof), which can measure the environmental gamma dose rate along the route.
However, neither ordinary nor energy-compensated Geiger–Müller counters are able to identify which radionuclides the count or radiation dose comes from. Therefore, even though a Geiger–Müller counter measures a higher than normal ambient radiation level, we still need other gamma radiation detector to determine which radionuclides in the environment are responsible for the higher radiation level.
Gamma Spectroscopy Detector
Natural or artificial radionuclides will display their unique energy peaks on the gamma energy spectrum. Therefore, by analyzing the gamma energy spectrum, we can know which gamma radionuclides are present. Scintillation detectors and semiconductor detectors are common gamma spectroscopy detectors.
(1) Scintillation Detector
A scintillator is a substance that emits fluorescence after being irradiated by ionizing radiation (such as X-ray or gamma ray). The operating principle of a scintillation detector is that when gamma ray is absorbed by the scintillator, photons are emitted, then based on photoelectric effect the photocathode generates photoelectrons, and then the photomultiplier tube multiplies these photoelectrons, which are finally converted into electronic pulses. If the energy of the absorbed gamma ray is higher, the resulting electronic pulse will be stronger. Therefore, scintillation detector is able to resolve the energy of incident gamma rays. Thallium-doped sodium iodide (NaI(Tl)) is a common scintillator and is generally referred to as sodium-iodide. Currently, the Observatory has also installed a "Sodium-iodide Gamma Spectrometer " on the roof of radiological survey vehicle (Figure 2: "Sodium-iodide Gamma Spectrometer " installed on the roof). Together with the "Gamma Dose-rate Meter", we can not only measure the environmental gamma dose rate but also the gamma energy spectrum, allowing us to "see" which gamma radionuclides are present along the route. When we use Sodium-iodide Gamma Spectrometer to measure gamma energy spectrum in natural environment, the energy spectrum can clearly show the energy peak of the natural gamma radionuclide "potassium-40" (Figure 3).
(2) Semiconductor Detector
Semiconductor detector is another common gamma spectroscopy detector. Since radioactive decay is a random process, it is generally described by the Poisson distribution. The characteristics of the Poisson distribution bring uncertainty to the radiation measurement. To reduce the statistical uncertainty, it is necessary to increase the number of signals measured by the detector. On the other hand, when the temperature of semiconductor is above absolute zero, it is known from thermodynamics that there is always a chance of creating an electron-hole pair. Since the measurement principle of gamma rays also relies on the generation of electron-hole pair, if the temperature is too high, the energy spectrum analysis of the detector will be hindered due to heat-generated noise. The energy required for semiconductor detector, such as High Purity Germanium Detector (HPGe Detector), to produce electron-hole pair is smaller than the energy required for scintillation detector, such as sodium-iodide detector, to produce photoelectrons. Therefore, semiconductor detector can produce more signals and have a higher energy spectrum resolution at the same gamma ray energy. In laboratory, HPGe detectors are typically cooled to the operating temperature with liquid nitrogen. Besides, portable HPGe detector also uses electronic cooler, allowing us to perform gamma spectroscopy analysis outdoors without the need for liquid nitrogen (Figure 4).
Conclusion
We briefly discussed three types of gamma radiation detectors used in radiological surveys. They have different efficiencies and resolutions, allowing them to excel in different aspects of gamma radiation measurement: Geiger–Müller counter can conveniently measure the environmental gamma radiation level, sodium-iodide detector can quickly measure gamma energy spectrum, and portable high purity germanium detector can measure high-resolution gamma energy spectrum for detailed nuclide analysis outdoors without the need for liquid nitrogen. Since artificial gamma radionuclides (such as iodine-131 and cesium-137) do not appear in the natural environment, we can determine whether the survey site has been affected by nuclear event by measuring whether there is presence of artificial gamma radionuclides.

Figure 1 Hand-held energy-compensated Geiger–Müller counter can conveniently measure the environmental gamma radiation level. The reading is higher when the detector is placed above stone (right) than above grass (left), indicating that the stone contains more natural radionuclides.

Figure 2 The Observatory’s radiological survey vehicle uses "Gamma Dose-rate Meter" and "NaI Gamma Spectrometer" installed on its roof to measure the gamma dose rate and gamma energy spectrum of the environment along the route, and identify any artificial gamma radionuclides in the environment in real time.
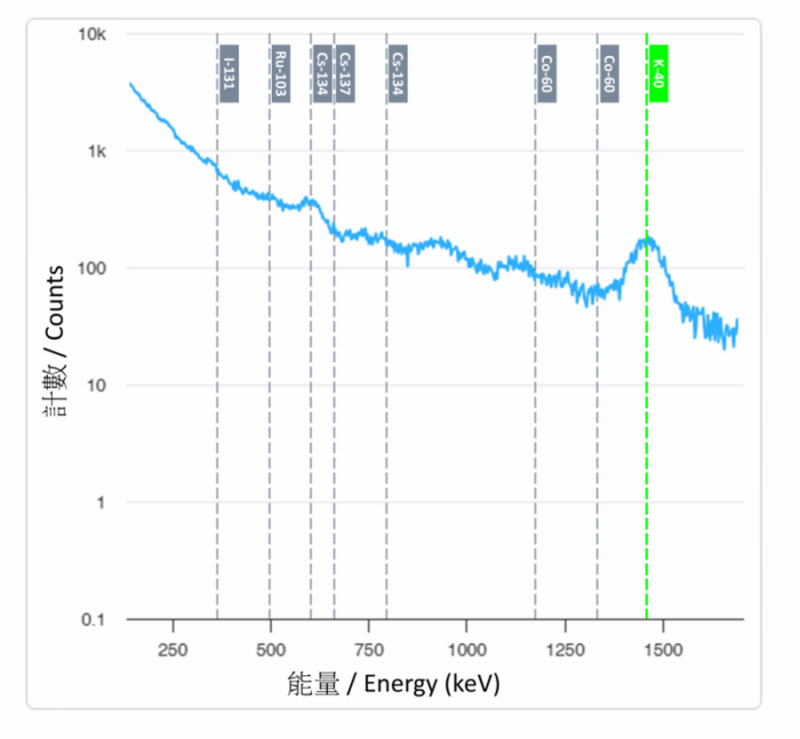
Figure 3 Gamma energy spectrum measured by Sodium-iodide Gamma Spectrometer in natural environment can clearly indicate the energy peak of the natural gamma radionuclide "potassium-40" and the absence of energy peaks of artificial gamma radionuclides such as "cobalt-60", "iodine-131", "ruthenium-103", "cesium-134" and "cesium-137".

Figure 4 Portable high purity germanium detector measures and analyzes radionuclides in the environment at survey site.
References:
[1] Hong Kong Observatory - Is there an antidote to radiation!?
[2] Hong Kong Observatory - Poisson Distribution and Radiological Measurement.
[1] Hong Kong Observatory - Is there an antidote to radiation!?
[2] Hong Kong Observatory - Poisson Distribution and Radiological Measurement.