Natural radiation
Radiation is everywhere in our environment. Even our body is also radioactive. We are therefore constantly exposed to different kinds of radiations, especially natural radiation. The annual dose received by the public in Hong Kong from natural background radiation is about 2 mSv. In general, the annual dose received by the public in the world is generally ranged from 1 mSv to 10 mSv
(Source:
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000 Report ).
Part of natural radiation received in daily life comes from space
Radionuclides, such as uranium-235, uranium-238, thorium-232 and neptunium-237, have been part of the earth since it came into existence. These radionuclides undergo radioactive decay, by emitting harmful alpha particles, beta particles or gamma rays. Their progenies are also unstable and radioactive. They will undergo radioactive decay until reaching a stable state. The half-lives of uranium-235, uranium-238, thorium-232 and neptunium-237 are 700 million years, 4.5 billion years, 14 billion years and 2.3 million years respectively. Since the half-lives of neptunium-237 and its daughter radionuclides are relatively short comparing to the age of the earth, these radionuclides are not present in the earth nowadays. On the contrary, radionuclides of the uranium-235, uranium-238 and thorium-232 series still exist in our environment. As soil and building materials contain these natural radionuclides, the dose we received therefore depends on the geological composition of the place we live and the building materials we use.
Radon (radon-222) is a major source of natural radiation. It is the decay product of uranium-238 in soil and rocks. Being a gas, part of it escapes from the ground into the atmosphere. If ventilation is poor, radon will accumulate in indoor areas. When we breathe in radon, the alpha particles emitted in the decay process will cause damage to our lung tissue. For the sake of our health, sufficient ventilation is required to reduce the amount of radon in our home.

Uranium ore
Another source of natural radiation is cosmic rays from space. Due to the shielding effect of the atmosphere, intensity of cosmic rays increases with altitude. The cosmic rays are mainly made up of high energy protons, plus some helium nuclei and heavy charged particles and ions, with atomic number equal to or greater than 3. When cosmic rays enter the atmosphere, they interact with nitrogen, oxygen and other atoms in the upper atmosphere and produce a large assortment of secondary particles, including radionuclides (such as tritium and carbon-14), neutrons, protons, electrons, mu(m) and pi(p) mesons and etc. Of them, carbon-14 is commonly used in assessing the age of antiquities.
Radionuclides are also present in our body. Potassium-40, uranium, thorium, radium, carbon-14, tritium and polonium are some examples. Our daily foodstuff consists of trace of radionuclides. These radionuclides will be absorbed through digestion and become part of our body. Meanwhile, their amounts will decrease due to decay or excretion. When the amounts of ingested and excreted radioactive materials reach an equilibrium, a constant level of radiation will be achieved in our body.
Radioactive decay series
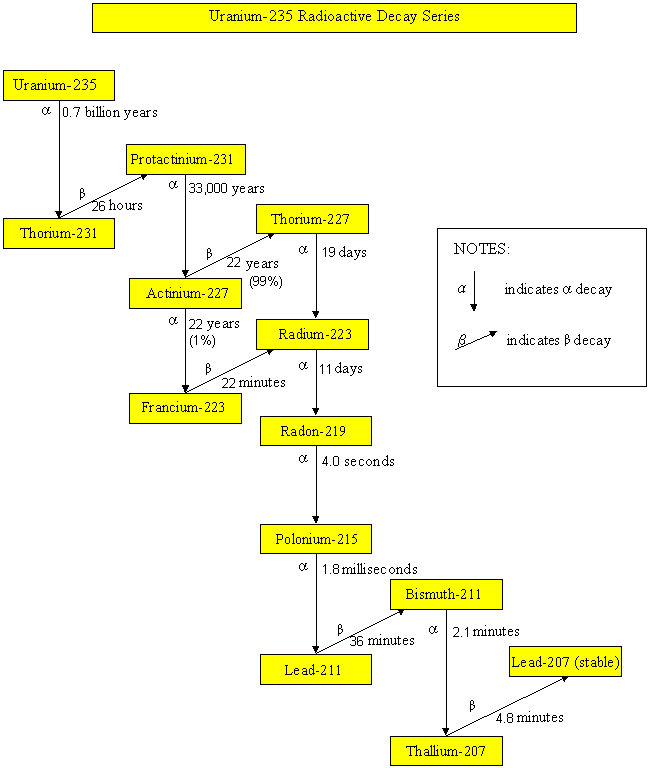
Uranium-235 Radioactive Decay Series
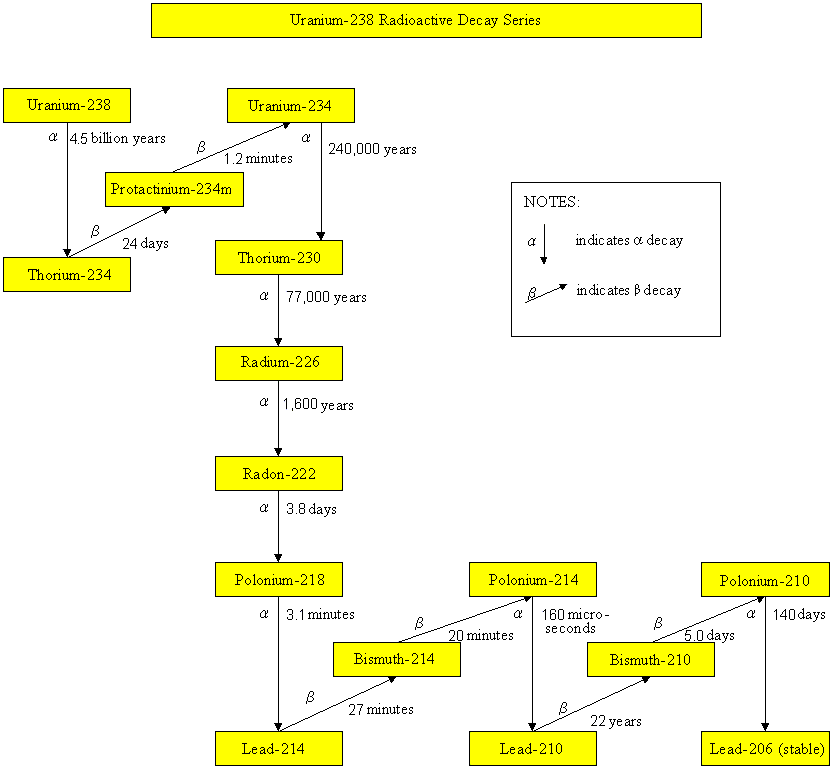
Uranium-238 Radioactive Decay Series
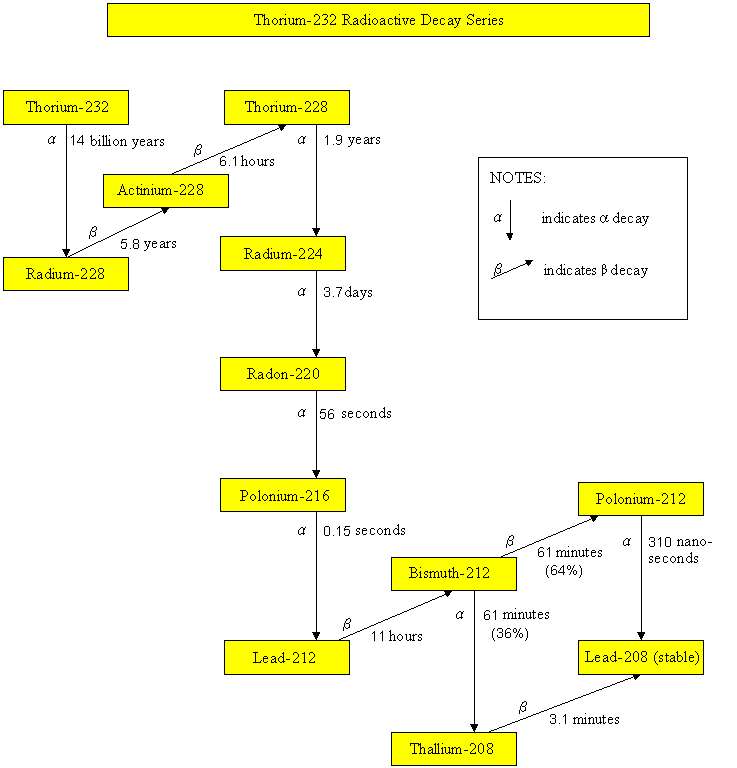
Thorium-232 Radioactive Decay Series