The Phantom of the Atoms - Alpha Radiation
The Phantom of the Atoms - Alpha Radiation
CHEUNG Sai-kit
September 2010
This article is about how ionizing radiation is produced from radioactive decay. To learn more about the basics of ionizing radiation, please visit the Hong Kong Observatory website.
Alpha (α) decay is the disintegration of a heavier nucleus (with higher atomic number) into a lighter nucleus (with lower atomic number) through the emission of an alpha particle. The emitted alpha particle (or alpha radiation) is a Helium-4 nucleus with two neutrons and two protons. Nearly all of the Helium in the atmosphere of the Earth is the by-product in alpha decay of heavy radionuclides like Uranium-238 and Thorium-234. There are two natural forces that are relevant to an alpha decay in a nucleus. The first one is the electromagnetic repulsive force between the protons in the alpha particle and those in the rest of the nucleus, which causes the instability of a nucleus. The second one is the 'strong' attractive force1 that confines the nucleons (protons and neutrons) to a small core. The balance between these two forces comprises a well-like nuclear barrier that forbids an alpha particle to leave the nucleus, just like a rigid impenetrable wall (see the diagram below).
Normally, an alpha particle in the nuclear well does not have enough energy to overcome the barrier. Here comes the puzzle - if an alpha particle cannot overcome the barrier, how can it escape from a nucleus in an alpha decay? This problem was solved by the theoretical physicist George Gamow in 1929 by using quantum theory. The answer is that the alpha particle can penetrate the wall! In the microscopic world, a particle is able to pass through a barrier. This strange phenomenon is known as 'quantum tunneling effect'2, and the probability of occurrence of this effect is tiny yet non-zero! As an alpha particle in a nucleus repeatedly bounces against the repulsive barrier at very high frequency, ultimately it will escape from the nucleus. Notwithstanding the small tunneling probability, it does not take long to observe a radioactive decay because there are already an enormous number of radionuclides in a small radioactive substance. For example, there are almost 2.5x1018 nuclei in each mg of Uranium-238 substance, thus on average there are 0.4 billion disintegrations in one second!
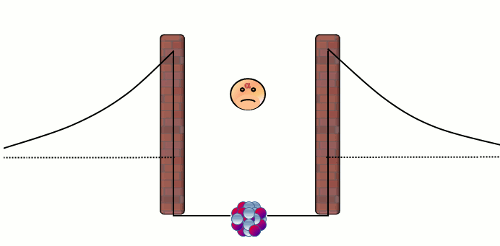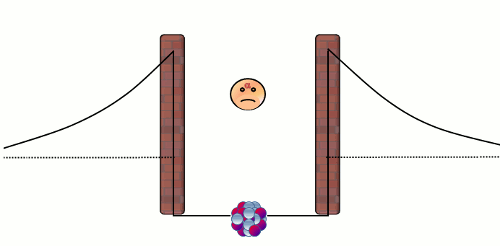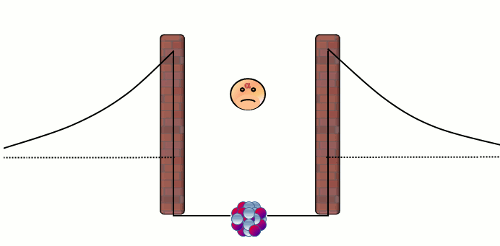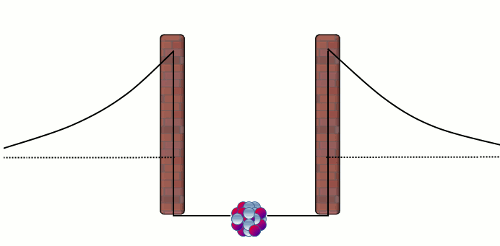
Figure 1: A trapped alpha particle repeatedly bounces against the wall of the nuclear barrier. The alpha particle has a non-zero probability to pass through the barrier, and thus eventually it will escape from the nucleus.
Remarks:
[1] The 'strong' attractive force here refers to the strong nuclear force, which is the strongest among the four fundamental forces in nature. It is 60 times stronger than the electromagnetic force in a nucleus but its range of influence is also much shorter.
[2] The occurrence of quantum tunneling relies on the wave nature of a particle. According to quantum mechanics, the probability to find a particle in any region is proportional to the integration of the squares of wave amplitude in that region. As the quantum wave of an alpha particle in a nucleus extends to the region outside the barrier, the probability to find the alpha particle outside is not zero.
[1] The 'strong' attractive force here refers to the strong nuclear force, which is the strongest among the four fundamental forces in nature. It is 60 times stronger than the electromagnetic force in a nucleus but its range of influence is also much shorter.
[2] The occurrence of quantum tunneling relies on the wave nature of a particle. According to quantum mechanics, the probability to find a particle in any region is proportional to the integration of the squares of wave amplitude in that region. As the quantum wave of an alpha particle in a nucleus extends to the region outside the barrier, the probability to find the alpha particle outside is not zero.
References:
[1] "Alpha decay", Wikipedia.
[2] "The New Quantum Universe", by T. Hey and P. Walters, Cambridge University Press, 2003.
[3] "Introductory Nuclear Physics", by K.S. Krane, Wiley, 1987.
[4] "Introduction to Nuclear and Particle Physics", by A. Das and T. Ferbel, John Wiley and Sons, 1993.
[1] "Alpha decay", Wikipedia.
[2] "The New Quantum Universe", by T. Hey and P. Walters, Cambridge University Press, 2003.
[3] "Introductory Nuclear Physics", by K.S. Krane, Wiley, 1987.
[4] "Introduction to Nuclear and Particle Physics", by A. Das and T. Ferbel, John Wiley and Sons, 1993.