Mixing Cloud in Winter
Written by: LEE Boon-ying
This relates to the mixing together of two different masses of moist air. To explain the phenomena, let's go back to some high-school science.
Recall the relationship between air temperature and vapour pressure (i.e. water vapour pressure). It looks something like the equilibrium curve in Figure 1 below.
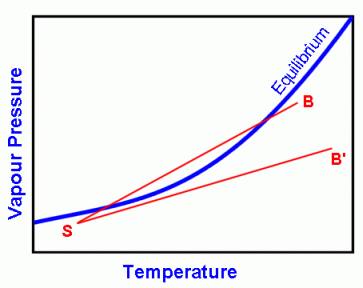
Figure 1
If a point representing an air mass lies below the equilibrium curve, e.g. point S, the air is said to be unsaturated. If it lies above the curve, the air is said to be supersaturated. If it lies on the curve, the air is said to be saturated.
In our example here, we have two air masses: one is represented by B (my breath, which is warm) which is almost saturated, and the other by S (surroundings, which is cooler) which is not saturated. The result of mixing these two air masses can be around anywhere on the straight line joining B and S. Notice that part of the line lies above the equilibrium curve, i.e. supersaturated.
Thus, two air masses that are themselves unsaturated may mix to produce a supersaturated air mass, and a cloud (which is just condensed water droplets) usually forms.
A cloud may not always form, however. For some other air masses, such as B' in Figure 1, when neither of the two air masses is moist enough, the straight line connecting it to S lies below the equilibrium curve. Under this situation, a cloud cannot form.
You'll notice that the equilibrium curve in Figure 1 curves upward, that is, the equilibrium vapour pressure increases faster at higher temperatures. If the curve were a straight line, clouds would never form when two unsaturated air masses mix, irrespective of how different they are.
Can you think of other instances of mixing? See here.