Bubbles in a soft drink (Part II)
Bubbles in a soft drink (Part II)
LEE Lap-shun
Why does the bubble grow?
When a bubble rises, it will experience less pressure in the surrounding liquid and therefore become larger. However, the change of pressure is very small in view of the short path from the bottom of the glass to the liquid surface. Therefore, pressure change is not the major cause of bubble growth.
Regarding the main cause, do you still remember that in the last issue we explained the role of nucleation sites in bubble growth? It is interesting to note that, besides the small cracks in the glass and the impurities inside the drink, the bubble itself in its ascent also acts as a nucleation site for carbon dioxide in the drink. Newly formed bubbles join with the old one, resulting in a larger bubble. This process continues while the bubble rises, and the bubble grows bigger and bigger.
Why are the bubbles more and more spaced apart as they rise?
A bubble rises because its density is less than that of the soft drink. This buoyancy increases as the bubble grows (Archimedes' Principle). On the other hand, the bubble experiences friction as it rises in the liquid and the resulting downward drag increases as the bubble grows. Although these two opposing forces increase at the same time, buoyancy increases more rapidly than the downward drag. As a result, the bubble will continue to accelerate in its ascent, leading to an increase in spacing between bubbles as they go further up.
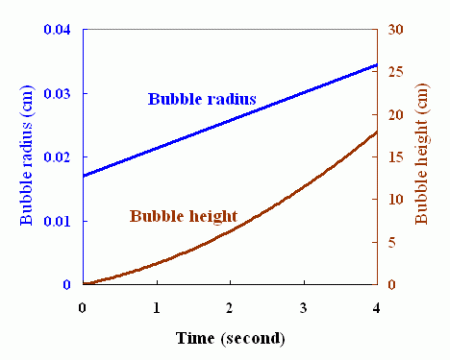
Increase of the bubble radius and bubble height with time, as revealed by experiments conducted elsewhere.
Remark: If you want to know more about this topic and look for a mathematical solution, please read: Shafer, Neil E.; Zare, Richard N., October 1991: Through a beer glass darkly. Physics Today, Volume 44, Issue 10, pp.48-52