Why carbon dioxide is a greenhouse gas?
Solar radiation reaches the Earth in the form of shortwave radiation and heats up the Earth's surface. The Earth’s surface then emits infrared radiation (longwave radiation) in order to cool down. However, greenhouse gases in the atmosphere will absorb part of the infrared radiation emitted by the Earth, and then re-emit infrared radiation in all directions. Part of the infrared radiation will escape to space but part of it will go back to the Earth, heating up the Earth's surface and causing the greenhouse effect.
The Earth's atmosphere consists of nitrogen (~78%), oxygen (~ 21%), argon (~0.9%) and trace gases such as carbon dioxide, water vapour, methane, etc. Nitrogen and oxygen together account for more than 90% of the atmosphere but they are not greenhouse gases. Carbon dioxide accounts for just about 0.04% but it is the single most important greenhouse gas in the atmosphere and also the principle control knob of Earth's temperature. Why carbon dioxide is a greenhouse gas? To answer this question, we first need to understand that infrared radiation is an electromagnetic wave. Laws of physics require that molecular vibration must lead to a change in the relative distribution of charge for the gas to absorb electromagnetic radiation.
Let us take nitrogen as an example for illustration. A nitrogen molecule consists of two nitrogen atoms (blue balls, left side of Figure 1). The positive charges carried by the two nitrogen atoms are identical and symmetrical in distribution. The vibration of nitrogen atoms along the chemical bond does not change the relative distribution of charges (left side of Figure 1). Therefore, nitrogen cannot absorb infrared radiation. Then how about carbon dioxide? A carbon dioxide molecule consists of two oxygen atoms (red balls, right side of Figure 1) and one carbon atom (grey balls, right side of Figure 1). Three different modes of vibration are possible with such configuration (right of Figure 1). When the carbon atom moves along the chemical bond towards either one of the oxygen atoms, or moves up and down relative to the oxygen atoms, the relative distribution of the charges will be altered and hence carbon dioxide can absorb infrared radiation.
Carbon dioxide is the single most important greenhouse gas in the atmosphere. The annual mean concentration set a record high again in 2017, reaching 405.5 ppm which is about 46% above pre-industrial levels. A recent study showed that the present-day atmospheric concentration of carbon dioxide is likely the highest in the last three million years, which is a worrisome situation indeed.
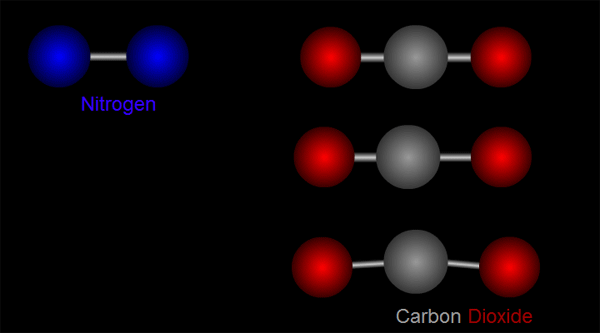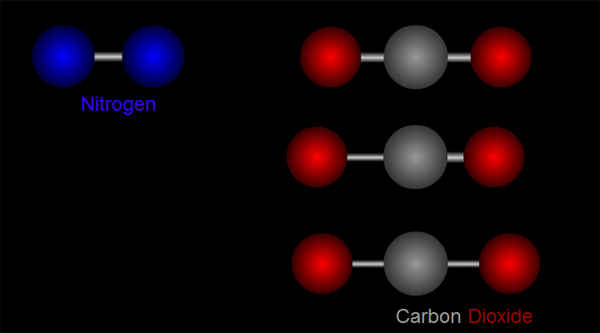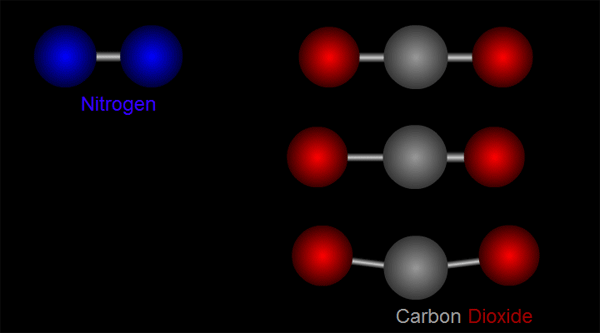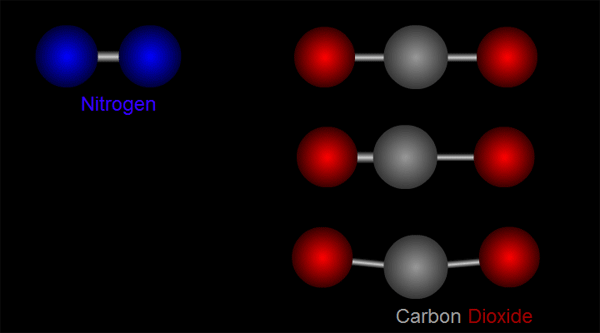
Figure 1 Modes of vibration of nitrogen and carbon dioxide molecules